-
OPEN positions!
We are looking for a person with experience in bioinformatics, computational biology, or data analytics.
If interested, please email: linas.mazutis at bti.vu.lt
Our current research topics include:
|
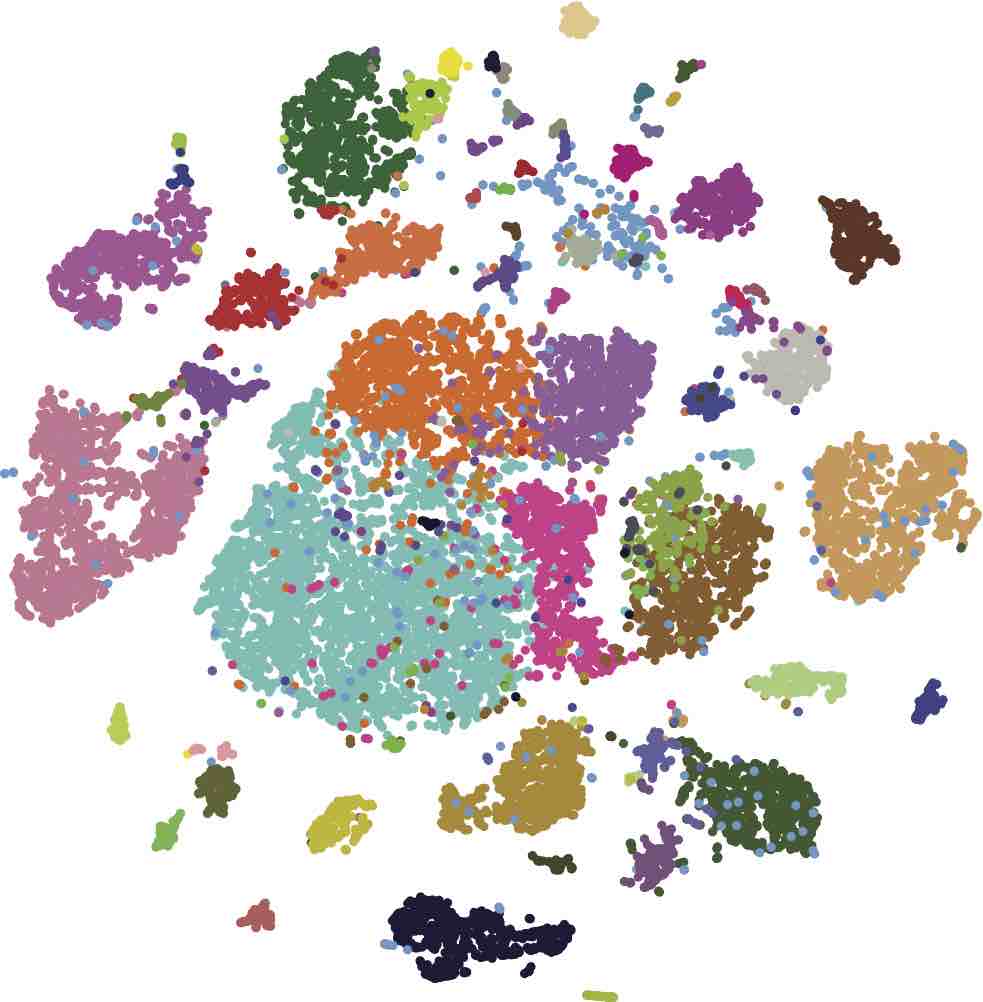 |
1. Single-cell transcriptomics, genomics, and epigenomics
Simultaneous profiling of the genome, epigenome, and transcriptome of the same cell has been a dream of many biologists for decades. Technological advances over the last few years have brought us closer to this reality by enabling single-cell transcriptomics, epigenomics, methylomics, and genomics. However, the integration of these individual modalities into a unified single-cell multi-omics profiling has proven a significant challenge. Although the latest developments have achieved dual-omics (joint profiling of the transcriptome and epigenome), the ultimate goal in the single-biology field is to integrate all layers of genetic and epigenetic information within individual cells. Simultaneous -omics studies on millions of single cells are especially challenging, not only due to technical difficulties associated with clinical sample processing and efficient single-cell isolation, but also to complex, multi-step, and sequential biochemical reactions that must be conducted on minuscule amounts of biomaterial. To address this challenge, we are developing new molecular barcoding tools to simultaneously profile the multi-ome (genome, epigenome, and transcriptome) of the same cell at high throughput and at an affordable cost.
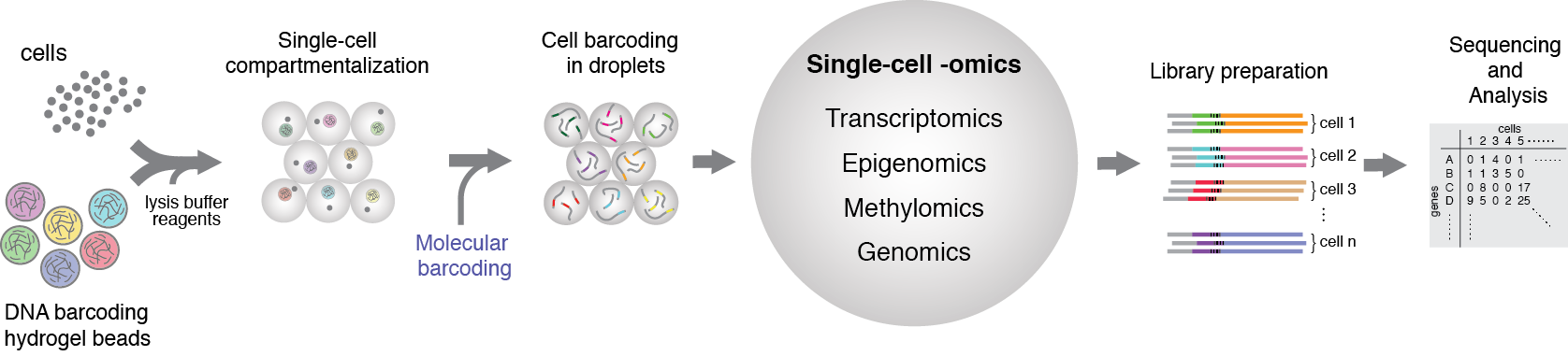
Figure 1. General schematics of the single-cell multiome approach that we are currently developing in our group. The idea is based on the isolation of individual cells in microfluidic droplets, together with suitable reagents for molecular barcoding of the transcriptome, epigenome, genome, and methylome. By leveraging the unique features of microfluidics technology and applying suitable biochemical reagents, it is possible to achieve indexing of individual cellular modalities simultaneously.
2. Single-cell barcoding and sequencing using droplet microfluidics
Deciphering the unbiased composition of heterogeneous cell populations requires innovative techniques that can capture not hundreds but thousands of single cells, in a high-throughput manner and at an affordable cost. This work is based on the idea of isolating individual cells into microfluidic droplets that carry RNA/DNA barcodes and assay reagents. Because individual cells are compartmentalized in drops, the cells' genetic makeup and contents can be accessed. For single-cell studies, our approach provides unprecedented scalability, significantly increased throughput, minimal sample loss, reagent savings, and considerable experimental flexibility. Using this technology, we are interested in understanding the genetic and epigenetic factors underlying cellular heterogeneity and inheritance.
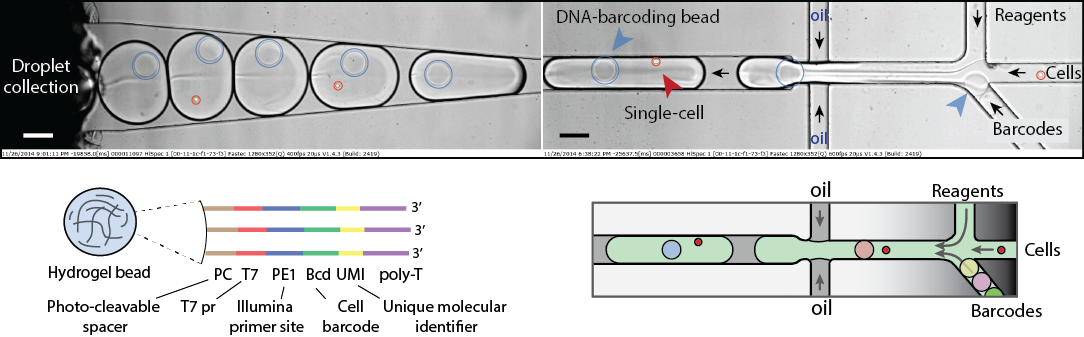
Figure 2. Single-cell transcriptomics. A heterogeneous mix of cells is encapsulated in droplets along with reverse transcription enzyme, a lysis mix, and hydrogel beads carrying barcoding primers. After encapsulation, cells are lysed, primers are released from the beads, and cDNA is tagged with a barcode during the reverse transcription reaction. Once cDNA synthesis is complete, droplets are broken, and barcoded material from all cells is amplified for next-gen sequencing. Read to learn more
3. High-throughput screening of antibody-secreting cells
In this project, we use a droplet microfluidics platform for high-throughput single-cell screening of cells that produce therapeutic antibodies or other biomedical biomolecules of interest. Cell compartmentalization into microfluidic droplets, together with capture beads and barcoded DNA primers, enables us to directly establish the linkage between the genotype (genes or mRNA) and the phenotype (binding, regulation, or activity of secreted proteins). Due to increased local concentration inside droplets, the amount of secreted antibodies will significantly reduce the time window necessary for the assay. We aim
to develop a discovery pipeline for quantitative, high-throughput antibody phenotyping that preserves the original heavy-light chain pairing,
a significant advantage over other technologies. Our technological approach provides a unique way to identify the primary sequences of heavy- and light-chain IgG genes encoding functional monoclonal antibodies directly from single cells, without the need for gene cloning, hybridoma construction, or cell immortalization.
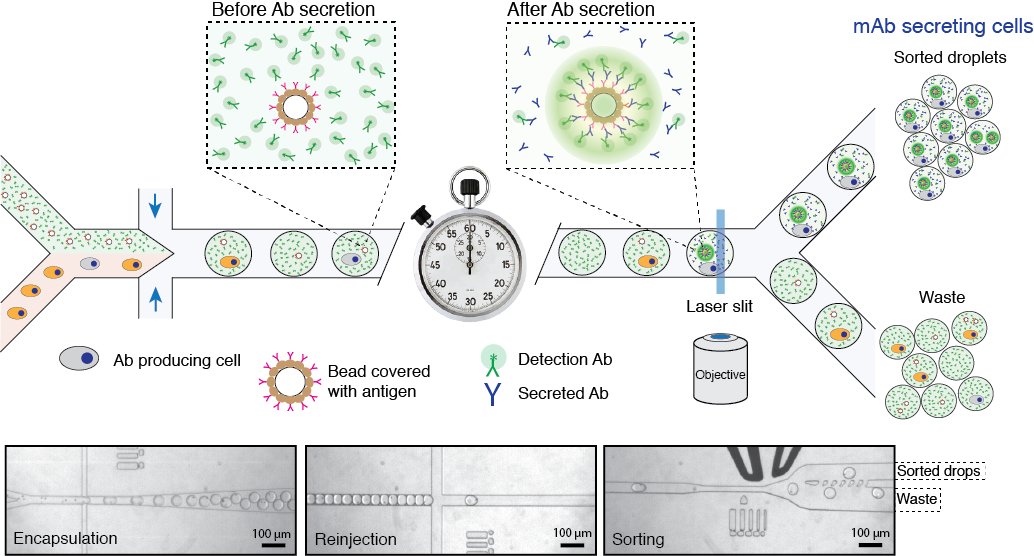
Figure 3. Schematics of a microfluidics platform for screening and sequencing single cells. A mixture of cells is loaded into droplets together with assay reagents and beads carrying a capture probe (antigen). The cells secreting antigen-specific Ab will be detected by a fluorescent sandwich assay on the bead surface and sorted. The sorted cells will be lysed, and the mRNA encoding heavy and light chains will be captured and sequenced. Alternatively, the sorted cells can be recovered directly into a growth medium for further cultivation, propagation, and analysis. Read to learn more
4. In vitro directed evolution using artificial cells
Darwinian evolution is a robust algorithm that has given rise to the functionally diverse set of proteins present in all living systems. Repetitive rounds of mutation, selection, and amplification have optimized nature’s catalysts, the enzymes, to an extraordinary degree, for example. Biocatalysts are typically much more efficient than their man-made counterparts, often working close to the diffusion limit. A better understanding of how new enzymes evolve consequently remains an essential and challenging task for both academic and industrial needs. Although the active sites of natural enzymes are highly complex, making the design of new or promiscuous protein functions difficult, computation has recently emerged as a potentially powerful strategy for creating protein catalysts with tailored activities and specificities. Because the activities of such artificial enzymes are still modest, we plan to explore the feasibility of optimizing them by combining the latest advances in droplet-based microfluidics technology with the methods of directed evolution. Specifically, two complementary groups, one with expertise in microfluidic systems and another with expertise in directed protein evolution, will join forces to investigate mechanisms and strategies for optimizing several computationally designed esterases. By applying droplet-based microfluidics
, in which biochemical reactions are performed inside micrometer-sized vessels, we will have a powerful tool enabling large numbers of libraries (>10^6) to be screened at ultra-high-throughput rates under conditions
incompatible with in vivo systems.
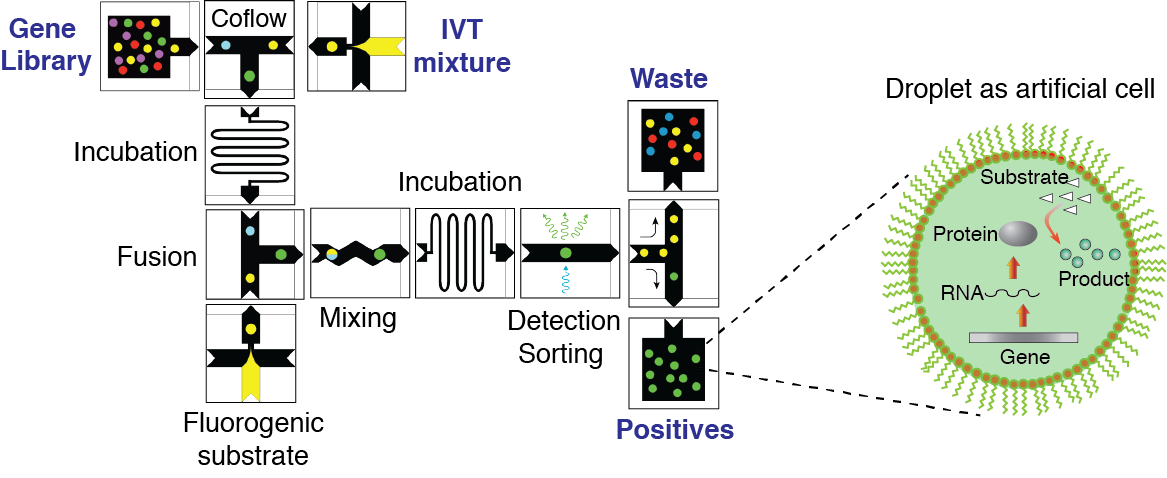
Figure 4. Concept of directed evolution approach in droplet microfluidics. Droplets containing single genes, with all the ingredients necessary for in vitro expression, will serve as artificial cells that can be selected for a desirable phenotype under conditions not feasible in living systems. The library of mutated genes is encapsulated so that, on average, each droplet contains no more than one gene. After the IVTT reaction, the droplets are fused with a second type of droplet containing a fluorogenic substrate and other molecules, salts, and reagents needed to impose the selection pressure. After mixing and incubation, the fluorescence of each droplet is recorded. Droplets with fluorescence above a threshold are sorted by means of dielectrophoresis, and the genes contained therein are amplified using PCR. The selected genes can be either characterized, re-selected, or mutated for a subsequent round of directed evolution.
5. Droplet microfluidic tools for biological and biomedical applications
Our group has a long history of experience in developing novel microfluidic tools for the precise manipulation and analysis of biological reactions at pico- and nanoliter volumes. We design, model, fabricate, characterize, and implement microfluidic systems and devices for a myriad of biological and biomedical applications for our colleagues and industrial partners. We build experimental platforms by integrating microfluidics with mechanical, electrical, and optical modules, enabling precise control over experimental conditions that are otherwise hard to achieve in bulk. Using droplet microfluidic devices, we provide significantly reduced assay volumes for virtually any biological assay and, as a result, significant cost savings for biochemical reagents and compounds. We are developing microfluidic devices and chips for digital DNA/RNA analysis, single-cell, and biomedicine applications.
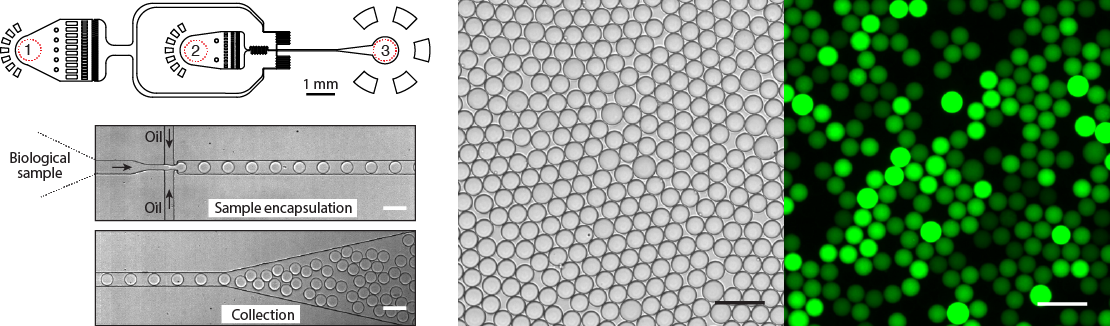
Figure 5. Design and operation of the droplet microfluidics device. Almost any biological sample (DNA, cells, proteins) can be encapsulated into picoliter- or nanoliter-volume droplets at >10.000 droplets per second. A typical microfluidics device has (1) the inlet for the continuous phase, (2) the inlet for the biological sample, and (3) the droplet collection outlet. Bright field and fluorescence images of an emulsion after a DNA amplification reaction. Droplets containing amplified DNA exhibit green fluorescence, whereas droplets lacking a template are dark. Scale bars, 50 μm.
6. Bacterial genomics and transcriptomics
Single bacterial genomics holds great promise for improving our understanding of the microbial world, discovering new enzymatic activities, and identifying antibiotic resistance, among other applications. However, performing single genome amplification (SGA) of an individual bacterium in water-in-oil emulsions can be hindered by the preceding cell lysis step, leading to inefficient or non-uniform DNA amplification or, in the case of hydrogel beads, significant cell loss during gel solidification. Bacterial lysis steps can be particularly problematic for single-step isothermal nucleic acid amplification methods (e.g., multiple displacement amplification [MDA]) or working with gram-positive bacteria, which are known to be much more resistant to thermolysis. To circumvent these limitations, we have recently introduced a method for producing biocompatible capsules with a thin, semipermeable shell. The shell acts as a passive sieve — retaining large-molecular-weight compounds, such as genomic DNA, while allowing smaller molecules (such as proteins) to diffuse through. We use an aqueous two-phase system (ATPS) composed of dextran and acrylate-modified polyethylene glycol to generate biocompatible hydrogel particles and apply them for whole-genome amplification of gram-negative and gram-positive bacterial cells. Capsules readily support multiple pipetting steps during complex biochemical reactions and generate higher genome amplification yields.
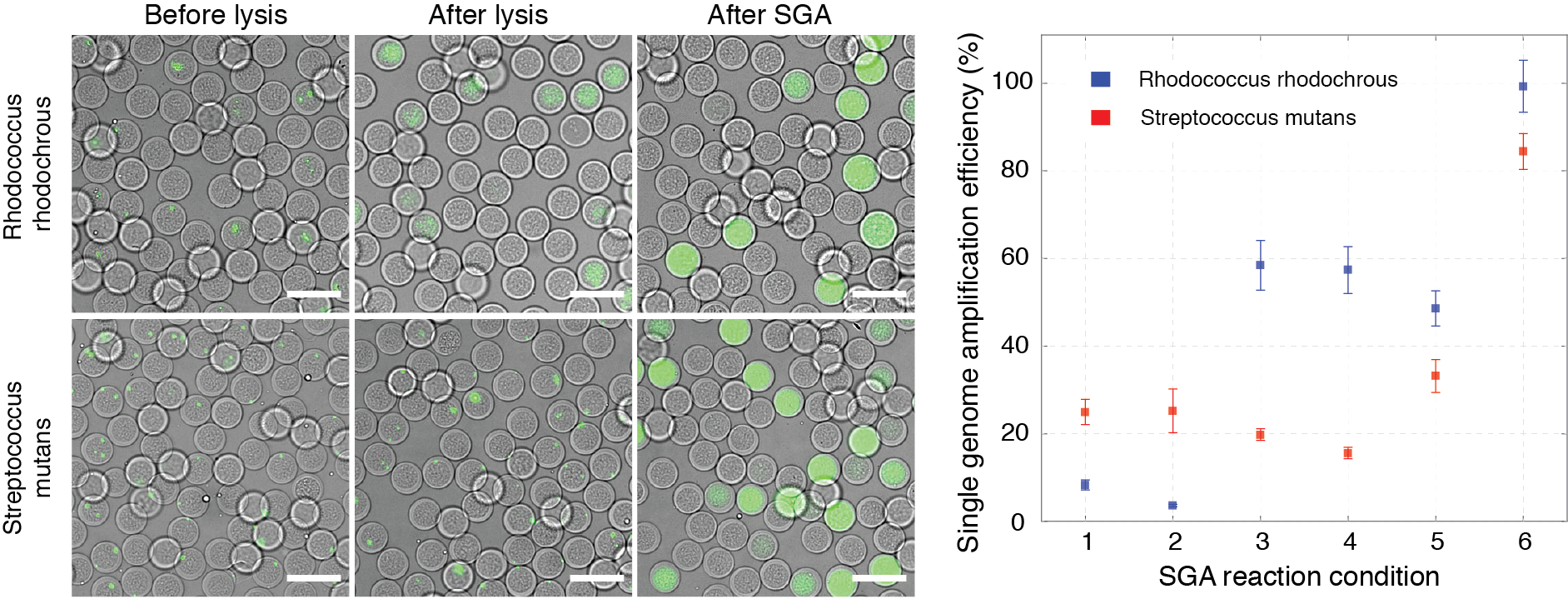
Figure 6.
Single-genome amplification from individual microbial cells is more efficient in semi-permeable capsules than in either hydrogel beads or droplets. Proteinase K and SDS treatment yielded the largest SGA on individual gram-positive cells using semi-permeable capsules. On the left, digital images showing capsules stained with DNA-binding dye (SYBR Green I) before lysis, after lysis, and after SGA. On the right, single-genome amplification efficiency is expressed as the percentage of observed over expected DNA amplification reactions for gram-positive bacteria lysed and processed under different conditions. Scale bars, 50 μm. Read to learn more
7. DNA supramolecular crystals and their use
Compartmentalization and amplification of single DNA molecules inside nanoliter- or picoliter-sized wells and droplets open new opportunities for biomedical and biological sciences. The most common method of amplifying DNA in a sample involves the polymerase chain reaction (PCR). Yet, amplification of long (>1 kb) single-molecule templates is often inefficient, leading to reduced reaction yields and biases. DNA amplification under isothermal reaction conditions has been shown to generate large amounts of material from a single-copy DNA template. Moreover, the ability to amplify DNA and express proteins from the clonally amplified template would significantly expand the potential applications. We employ droplet microfluidics devices to encapsulate and convert single DNA molecules into condensed DNA nanoparticles via multiple displacement amplification (MDA) driven by phi29 DNA polymerase. The condensed DNA nanoparticles comprise up to ~10.000-100.000 copies of clonally amplified DNA template. Intriguingly, we found that inorganic pyrophosphate, produced during isothermal DNA synthesis, and magnesium ions are prerequisites for DNA condensation into crystalline-like globular structures. This process is enhanced when a DNA amplification reaction is performed within droplets, which can be attributed to the confined volumes and spatial accumulation of reaction products. We have demonstrated the biological functionality of the DNA nanoparticles by applying them in in vitro transcription-translation reactions and observed improved protein expression yields relative to standard assay conditions.
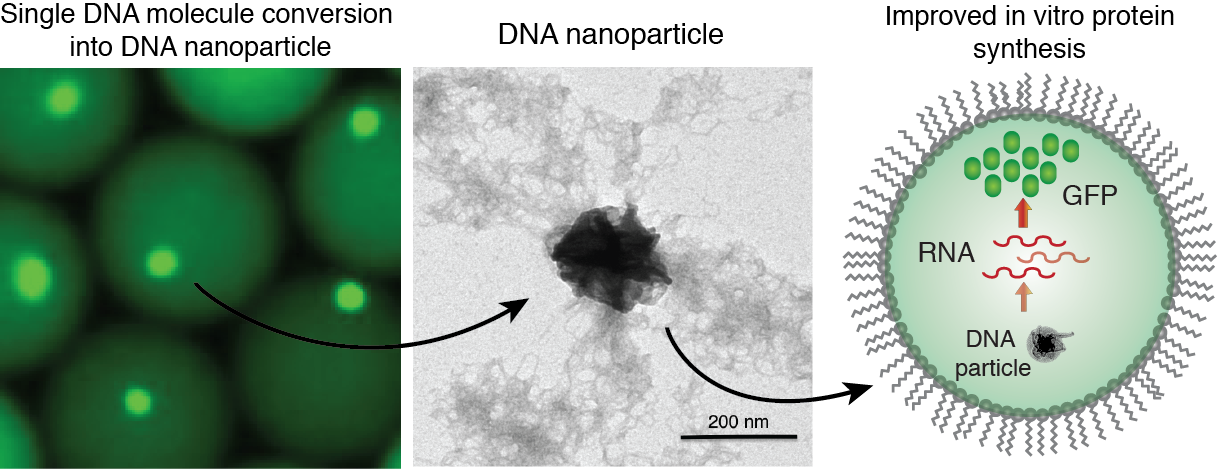
Figure 7. Generation of DNA-Mg-PP particles. DNA nanoparticle formation induced by inorganic pyrophosphate (PP) and magnesium ions during a phi29-catalyzed DNA polymerization reaction. TEM image of the DNA-Mg-PP particle. The use of DNA for efficient gene expression and protein synthesis in vitro. Read to learn more
