|
|
LILIJA KALĖDIENĖ Professor Head, Department of Microbiology and Biotechnology Institute of Biosciences Email: Phone: +370 5 239 8205 |
|
Although the potential for microbial degradation is ubiquitous, many organic contaminants are not or often only poorly transformed in natural environmental conditions, thus, organic, and other waste treatment and recycling is an important topic. Therefore, the enhancement of natural microbiological degradative activities at contaminated sites is one of the challenges of the present research group. Through exploitation of advances conventional and molecular biology techniques, search, identification, and characterization of microbial enzymes active towards fatty substances or aromatic compounds are done. Microbial enzymes, especially those exerting activity against ester bonds have a broad range of applications in modern biotechnology. Lipolytic enzymes are among the most industrially relevant and widely used in biocatalysis, both at academic and industrial levels due to their immense versatility regarding catalytic behaviour and great stability in different reaction media. Nevertheless, for the industrial implementations, immobilized enzymes are preferred over their soluble forms. If the enzyme is immobilized properly, it can be considered as a special type of formulation of its properties such as activity, stability, selectivity, purity, and others. Therefore, it is important to examine new types of immobilization sorbents. Ecologically inspired method of immobilization of lipolytic enzymes on industrial waste products as carriers are developed by the group.
Another emerging topic is alternative antibacterial compounds such as bacterial ribosomally synthesized peptides with antibacterial activity (bacteriocins). These natural coumpounds have considerable diversity with respect to their size, structure, mechanism of action, inhibitory spectrum, immunity mechanisms and targeted receptors. In the era of antibiotic resistance, bacteriocins are suggested as a potential alternative to antibiotics in clinics and as food preservatives against spoilage and pathogenic microorganisms.
The research group is also participating in a research regarding safe bacterial biofilm control method development for European Space Agency (ESA). In collaboration with the Institute of Photonics and Nanotechnology, Faculty of Physics (Vilnius University), a novel natural photosensitizers-based antimicrobial photoinactivation (API) technology that is safe for the use in the confined, closed-loop systems such as spacecraft is being developed.
Yeast β-glucans, a diverse group of polysaccharides, exhibiting immunostimulating activity, and algal pigments, which, besides their health benefits, have great commercial value in nutraceutical, cosmetic and pharmaceutical industries, are among the research group’s topics as well.
Enzymes and antimicrobial compounds and systems that are analysed by our research group, are attractive both biotechnologically and in basic research. Some of the competences are achieved not only by introducing publications but also by participating in scientific projects co-financed by ESA, EU funds and collaborating with the regional waste treatment company for the pilot study of biogas production from municipal waste.
Some of our PhD students defended doctoral theses describing the identification of new bacterial lipolytic enzymes and post-translationally modified bacteriocins.
SELECTED PUBLICATIONS
- Buchovec, I., Gricajeva, A., Kalėdienė, L., Vitta, P. Antimicrobial photoinactivation approach based on natural agents for control of bacteria biofilms in spacecraft. International Journal of Molecular Sciences. 2020, 21 (18): 6932.
- Kaunietis, A., Buivydas, A., Citavicius, D., Kuipers, O. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nature Communications. 2019, 10, Article No: 1115.
- Gricajeva, A., Bikute, I., Kalediene, L. Atypical organic-solvent tolerant bacterial hormone sensitive lipase-like homologue EstAG1 from Staphylococcus saprophyticus AG1: Synthesis and characterization. International Journal of Biological Macromolecules. 2019, 130: 253–265.
- Vaičikauskaitė, M., Ger, M., Valius, M., Maneikis, A., Lastauskienė, E., Kalėdienė, L., Kaunietis, A. Geobacillin 26 - high molecular weight bacteriocin from a thermophilic bacterium. International Journal of Biological Macromolecules. 2019, 141: 333–344.
Microbial biofilms are widespread in the environment and form on biotic and abiotic surfaces if constant moisture is present. Biofilms play an important role in human infections and endanger material integrity not only in confined facilities such as hospitals and food settings on Earth but also in spacecraft, which is inhabited by a changing microbial consortium mostly originating from life-supporting devices, equipment collected in pre-flight conditions and crewmembers. Resilient biofilms pose a higher risk to crewmembers’ health and the material integrity of the spacecraft than planktonic cells. Moreover, biofilms in space conditions are characterized by faster formation and acquisition of resistance to chemical and physical control methods than under the same conditions on Earth, making most decontamination methods unsafe. Thus, biofilm control methods that are safe for confined, closed-loop systems such as spacecraft are of high demand. Visible-light irradiation technology - antimicrobial photoinactivation (API) based on natural PSs such as riboflavin (RF) and chlorophyllin - are being developed by the research group, and the technology shows promising results for its use in the mentioned area (confined, closed-loop facilities such as spacecraft and others). API belongs to a multitarget process; therefore, bacteria do not develop resistance, moreover, the process exhibits a very rapid microbial killing.
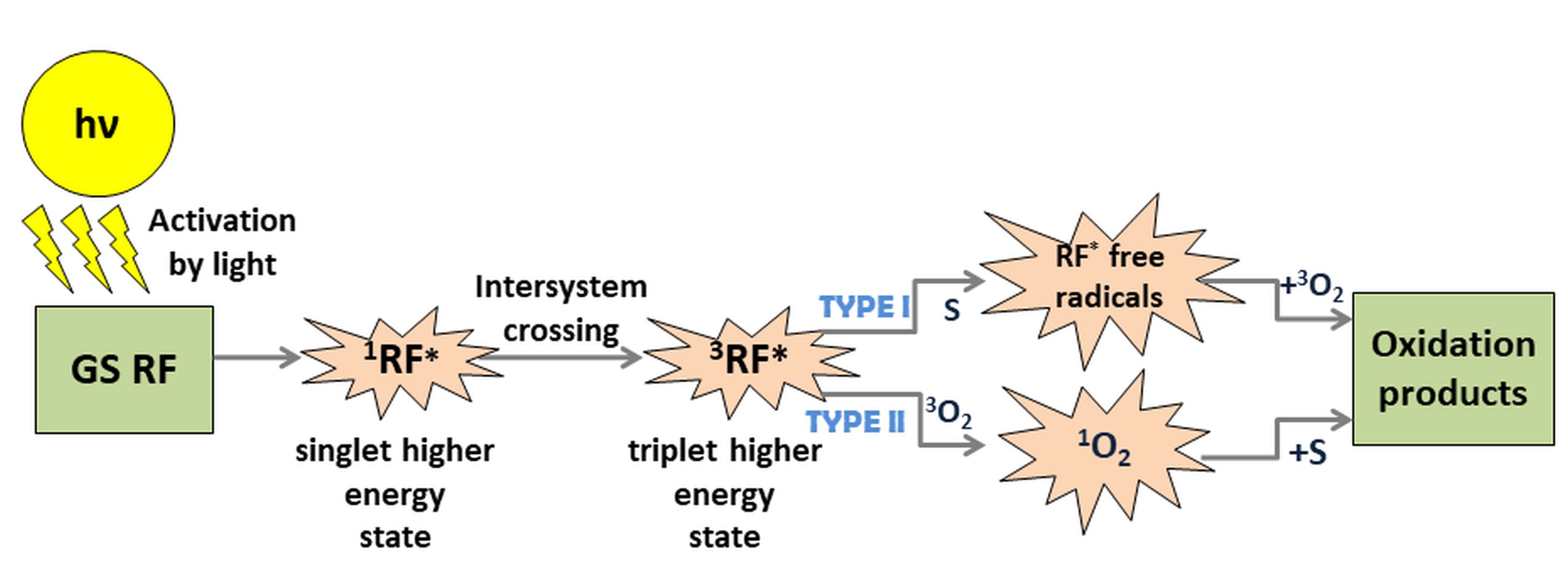
Fig. 1. Example of natural PSs-based API mechanism; upon activation by light, RF is excited to a singlet state of higher energy (1RF*), followed by intersystem crossing to an excited triplet state (3RF*). 3RF* can be involved in a photosensitized oxidation process of two types. Type I: RF transfers the energy to a substrate (S) and generates RF* free radicals interacting with molecular oxygen in the ground state (GS) to yield oxidation products. Type II: RF transfers the energy to molecular oxygen in the ground state to generate the more reactive 1O2. The process leads to the final products harmful to microbes.
Biocatalysts exerting activity against ester bonds have a broad range of applications in modern biotechnology. Some of the most industrially relevant enzymes of this type are lipolytic. A novel bacterial hormone-sensitive lipase-like (bHSL) family homologue, designated EstAG1, was discovered by mining gDNA of bacteria isolated from fat contaminated soil. EstAG1 was hyperactivated by organic solvents implicating that it could be an industrially applicable enzyme for the organic synthesis of valuable products such as biodiesel, flavour and aroma esters etc. Based on low EstAG1 amino acid sequence identities to closest homologues, by unique catalytic amino acid peculiarities in the sequence and phylogenetic analysis the enzyme belongs to a new family of bacterial lipolytic enzymes.
Fig. 2. Application possibilities of bacterial lipolytic enzymes.
The genome of the thermophilic bacterium, Aeribacillus pallidus 8, encodes the bacteriocin pallidocin belonging to a small class of glycocins and is posttranslationally modified by S-linked glucose on a specific Cys residue. In this study, the pallidocin biosynthetic machinery was expressed in E. coli to achieve its biosynthesis and modification. The characterized biosynthetic machinery was employed to produce two other glycopeptides Hyp1 and Hyp2. Heterologous expression of a glycocin biosynthetic gene cluster with S-glycosyltransferase provides a good tool for production of hypothetical glycocins encoded by various bacterial genomes and allows rapid in vivo screening.
Moreover, geobacillin 26 from Geobacillus stearothermophilus 15 was thoroughly investigated. Our study suggests that this bacteriocin is not a cell wall hydrolyser as most of high molecular weight bacteriocins and has no amino acid sequence similarities to other known function proteins. No other class III bacteriocin from a thermophilic bacterium has been reported and well characterized before.
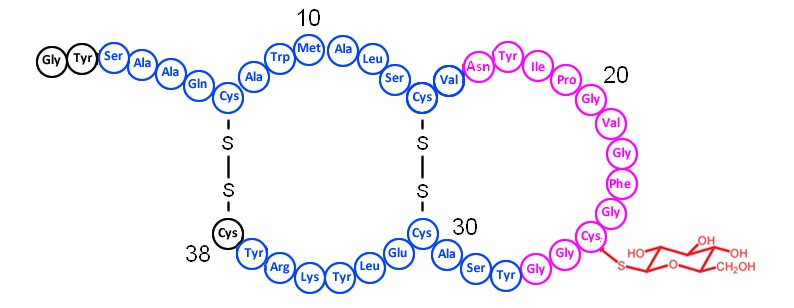
Fig. 3. Proposed structure of pallidocin. The α-helical structure shown in blue, coil structure in purple.

Fig. 4. Antibacterial activity of Geo26-His. NB-agar medium inoculated with sensitive strain P. genomospecies1 NUB36187.


