|
|
RŪTA NAVAKAUSKIENĖ Research Professor Head, Department of Molecular Cell Biology Institute of Biochemistry Email: Phone: +370 5 223 4409 |
|
Epigenetic regulation, when influenced by DNA and histone modifications as well as microRNA expression, causes variances in gene expression and cell phenotype. It has a great influence on the development and functioning of stem cells. These changes could cause cancer and other diseases. An understanding of regulatory and epigenetic molecular mechanisms of stem and cancer cell functioning is the main interest for developing new tools in regenerative medicine as well as novel epigenetic therapeutics. Many factors influence the regulation of stem cell, cancer stem cell and cancer cell proliferation, differentiation and apoptosis, including intracellular signalling molecules, transcription factors and epigenetic events. However, the epigenetic and other regulatory mechanisms, governing stem and cancer cell identity, as well as fate determination are still not well-understood.
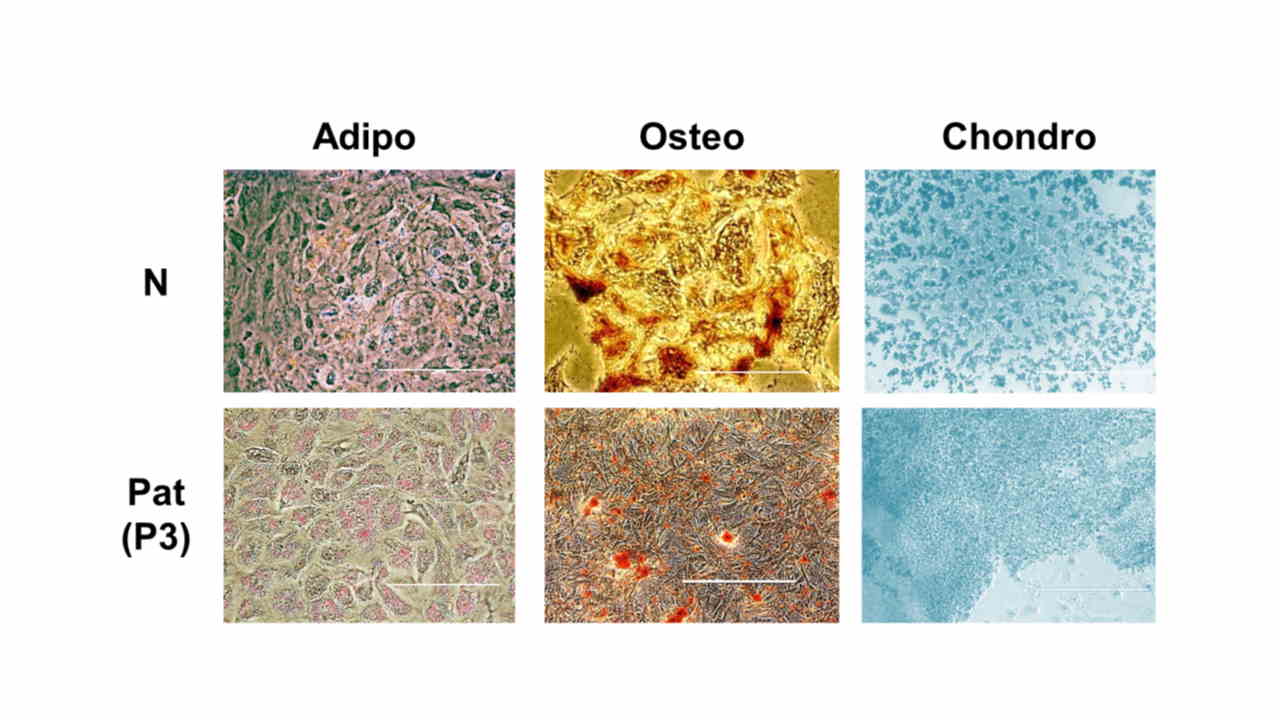
Human amniotic fluid-derived stem cells (AFSCs) are a valuable, easily obtainable alternative of stem cells for cell therapy and regenerative medicine. Although this field has gained much research attention, differentiation capacity of AFSCs and epigenetic regulation leading to AFSCs fate determination are still poorly characterized. Therefore, in our study we investigated the differentiation potential and assessed epigenetic factors involved in tissue-specific differentiation.
SELECTED PUBLICATIONS
- Zentelytė, A., Gasiūnienė, M., Treigytė, G., Baronaitė, S., Savickienė, J., Borutinskaitė, V., Navakauskienė, R. Epigenetic regulation of amniotic fluid mesenchymal stem cell differentiation to the mesodermal lineages at normal and fetus-diseased gestation. Journal of Cellular Biochemistry. 2020 Feb, 121(2): 1811–1822. doi: 10.1002/jcb.29416.
- Gasiūnienė, M., Valatkaitė, E., Navakauskienė, R. Long-term cultivation of human amniotic fluid stem cells: The impact on proliferative capacity and differentiation potential. Journal of Cellular Biochemistry. 2020 Jan 3. doi: 10.1002/jcb.29623.
- Gasiūnienė, M., Zentelytė, A., Treigytė, G., Baronaitė, S., Savickienė, J., Utkus, A., Navakauskienė, R. Epigenetic alterations in amniotic fluid mesenchymal stem cells derived from normal and fetus-affected gestations: a focus on myogenic and neural differentiations. Cell Biology International. 2019 Mar, 43(3): 299–312. doi: 10.1002/cbin.11099.
- Gasiūnienė, M., Zentelytė, A., Wojtas, B., Baronaitė, S., Krasovskaja, N., Savickienė, J., Gielniewski, B., Kaminska, B., Utkus, A., Navakauskienė, R. DNMT inhibitors effectively induce gene expression changes suggestive of cardiomyogenic differentiation of human amniotic fluid-derived mesenchymal stem cells via chromatin remodeling. Journal Tissue Engineering and Regenerative Medicine. 2019, 13: 469–481. doi: 10.1002/term.2800.
The Impact of AFSCs on Long-Term Cultivation Capacity and Differentiation Potential
It is crucial to investigate whether the main characteristics of SCs are stably maintained during the long-term cultivation in vitro and whether the passage number of AFSCs culture can have an impact on the future clinical applications. Therefore, we examined the proliferation, differentiation, death and senescence of amniotic-fluid derived stem cells during the long-term culturing in vitro. AFSCs were expanded up to 42 passages and maintained their stemness and mesenchymal characteristics. Alterations in morphology, the expression of pluripotency genes and cell surface markers concomitant with senescence initiation as well as reduced cardiomyogenic differentiation potential were detected at the late passages. These results provide useful insights into the potential use of AFSCs for bio-banking and universal applications requiring large amounts of cells or repeated infusions (Gasiūnienė, Valatkaitė & Navakauskienė, 2020).
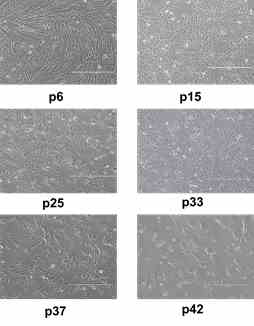
AFSCs morphology from different passages of long-term cultivation
Metabolic and Neurogenic Potential of AFSCs from Normal vs Foetus Affected Gestations
Although AFSCs are widely researched, their analysis mainly involves SCs obtained from normal foetus unaffected gestations. However, in clinical setting, the knowledge about AFSCs from normal gestations would be poorly translational, as AFSCs from normal and foetus diseased gestations may differ in their differentiation and metabolic potential. Therefore, in this study the metabolic, neurogenic and neurotrophic potential of AFSCs, obtained from foetus affected gestations with polyhydramnios, in comparison with foetus unaffected gestations, were investigated. Results demonstrated that these cells are similar in gene expression levels of stemness markers. However, they do differ in expression of certain cell surface markers. In addition, AFSCs from “Normal” and “Pathology” groups were found to be different in oxidative phosphorylation rate, as well as in the level of ATP and reactive oxygen species production. AFSCs from normal gestations were found to be more prone to neurogenic differentiation. Overall, these observations provide supplementary insights into the neurogenic potential of AFSCs obtained from foetus unaffected vs foetus affected gestations
(VU funded research No. MSF-LMT-3/2020).
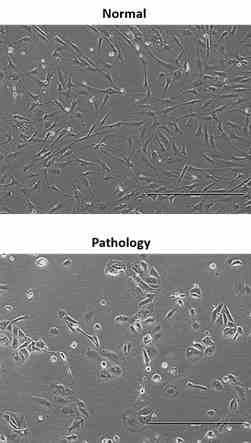
Morphology of AFSCs obtained from healthy and foetus diseased gestations upon neural differentiation induction