Tissue engineering or the fabrication of artificial tissue is a promising field of regenerative medicine, which meets a lot of scientific and technological challenges. Artificial tissues could be produced by using different biofabrication techniques, which depend on the specifics of the tissue being created.
Fabrication of an artificial biocompatible, osteoconductive and osteoinductive bone graft using special scaffold still remains a major issue in bone tissue engineering approaches. It is obvious that the newly produced bone graft should not only stimulate cells to regenerate damaged bone tissue, it also should mimic patient-specific bone defect morphology and should be low in price and high in production speed. One of the most important factors for constructing an artificial bone tissue is the morphology of the bone scaffold. It is known that the material and topography of the scaffolds have an impact on cell focal adhesions (FA) formation, which in turn modifies cell shape and morphology, leading to various signalling pathways activation and eventually influencing cell adhesion, proliferation, and differentiation.
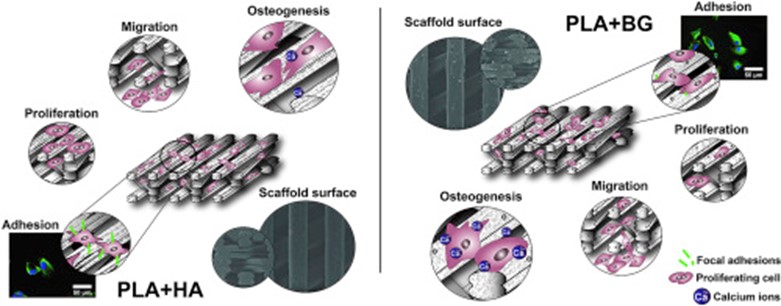
We have previously demonstrated that low-cost 3D printed polylactic acid (PLA) macro structures (larger than cell diameter) without additional surface modifications could promote spontaneous stem cell osteogenic differentiation. Currently, we aimed to improve the osteoinductivity of these scaffolds and attenuate the negative effects of PLA degradation by creating PLA composites with 10% of hydroxyapatite (HA) or 10% of bioglass (BG) filaments, which were further used for scaffold production.
The tasks of our group are (1) to develop the best way of selected composite material microstructurization, (2) to compare the physical and osteoinductive properties of 3D printed PLA+ hydroxyapatite (HA), PLA+bioglass (BG), (3) to elucidate the fate of cells grown on these scaffolds and (4) to evaluate the influence of biodecoration effect on cell differentiation.
We have analysed various fused filament fabrication (FFF) 3D-printed PLA scaffold modifications’ impact on the fate of rat dental pulp stem cells (DPSC). Primary rat DPSCs were selected as a model, which helped to understand the cellular response to different substrate modifications. PLA scaffolds were modified by altering their chemical composition (PLA composites with 10% of HA or PLA with 10% of BG), and surface coating with proteins to determine the impact of each substrate modification on cell function and properties, focusing on cell osteogenesis processes.
SELECTED PUBLICATIONS
- Alksne, M., Kalvaityte, M., Simoliunas, E., Rinkunaite, I., Gendviliene, I., Locs, J., Rutkūnas, V., Bukelskienė, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J Mech Behav Biomed Mater. 2020, 104: 103641.
- Gendviliene, I., Simoliunas, E., Rekstyte, S., Malinauskas, M., Zaleckas, L., Jegelevicius, D., Bukelskiene, V., Rutkūnas, V. Assessment of the morphology and dimensional accuracy of 3D printed PLA and PLA/HAp scaffolds. J Mech Behav Biomed Mater. 2020, 104:103616.
- Grigaleviciute, G., Baltriukiene, D., Bukelskiene, V., Malinauskas, M. Biocompatibility evaluation and enhancement of elastomeric coatings made using table-top optical 3D printer. Coatings. 2020, 10: 254.
- Han, Y., Baltriukiene, D., Kozlova, E. Effect of scaffold properties on adhesion and maintenance of boundary cap neural crest stem cells in vitro. J Biomed Mater Res A. 2020, 108: 1274–1280.
- Meškyte, E., Keskas, S., Ciribilli, Y. MYC as a multifaceted regulator of tumor microenvironment leading to metastasis. Int J Mol Sci. 2020, 21: 3234.
Impact of Extracellular Environment on Cell Fate
Analysis of substrate macrophotography (>100 µm) impact on the fate of DPSCs revealed that for spontaneous cell osteogenesis induction, the precise structuring of the scaffold (nano- and micro-surface patterns) is not necessarily required. Nevertheless, we have found that PLA+HA filaments printed with FFF 3D printer produced equal or even better accuracy than scaffolds printed with pure PLA filaments (Gendviliene et al. 2020). After evaluating the impact of surface chemical composition on cell behaviour it was observed that PLA+HA composite was more suitable for DPSC attachment and proliferation, meanwhile the minimum number of focal adhesions were formed in the cells grown on PLA+BG scaffolds, which means that DPSCs adhesion strength on PLA+BG scaffolds was the weakest compared with PLA+HA (p<0.05) and pure PLA (p<0.05) (Fig.1). Despite this, PLA+BG composites promoted the earliest and strongest DPSC osteogenesis.
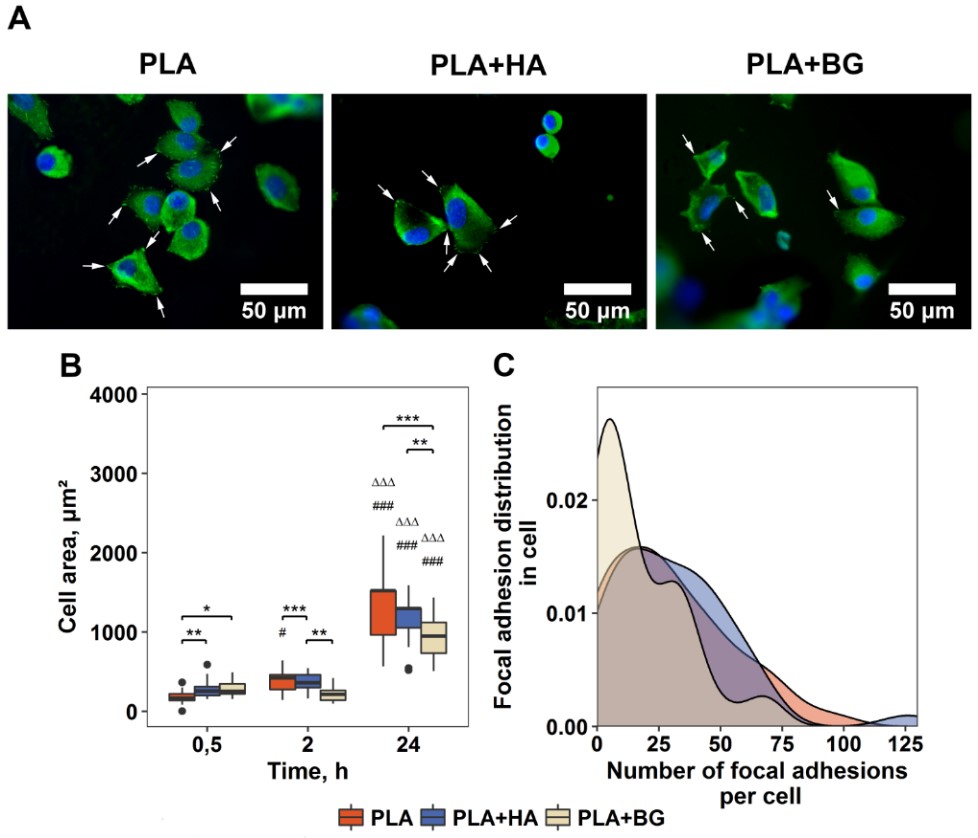
Fig. 1. DPSCs adhesion on 3D printed composite scaffolds. A – immunofluorescence staining of nucleus (DAPI, blue) and FA spots (vinculin, green) in DPSCs 24 h post-seeding; B – cell surface area after culturing for 0.5, 2 and 24 h on the scaffolds; C – quantitative FA evaluation within the cells after culturing for 24 h.
Further investigation has shown that PLA surface coated with DPSC-derived extracellular matrix (ECM) network significantly improved the osteoinductive properties of the surface. The obtained results of DPSC migration and proliferation showed that ECM proteins had a positive impact on both cell migration and proliferation (Fig. 2).
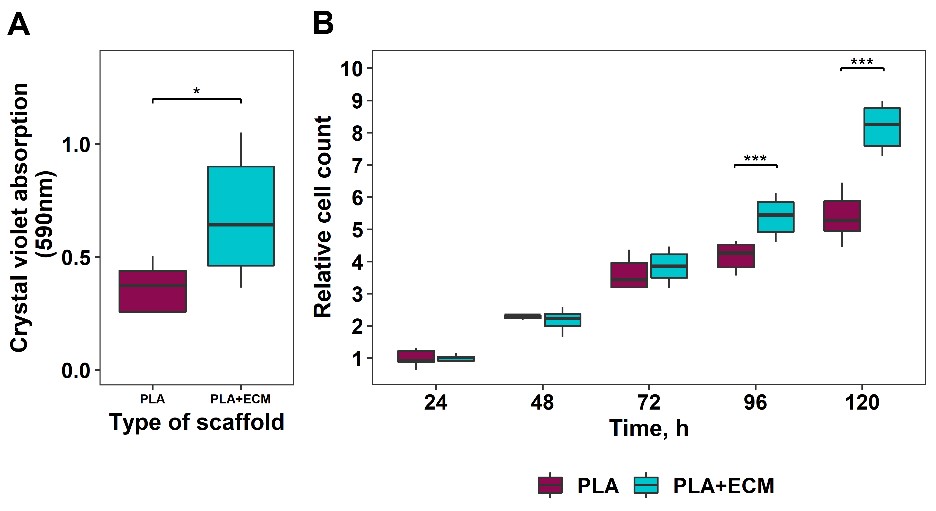
Fig. 2. DPSC migration and proliferation on ECM coated scaffolds. A – evaluation of vertical cell migration onto the scaffolds using crystal violet assay; B – relative DPSC proliferation rate.
The in vitro assay of scaffolds (PLA, PLA+HA, PLA+BG, and PLA+ECM) and DPSCs ability to initiate angiogenesis revealed that DPSC-PLA+BG and DPSC-PLA+ECM constructs would be the most suitable candidates for bone engineering. According to our data, the combination of DPSCs, PLA+BG composite microstructured scaffold and DPSC-derived ECM network can be expected to reach successful bone tissue regeneration in vivo (Alksne et al. 2020).
