|
|
NARIMANTAS ČĖNAS
|
|
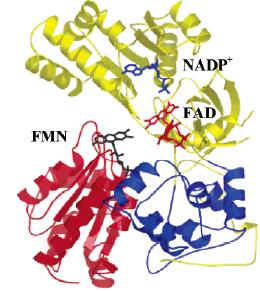
Flavoenzymes contain flavinmononucleotide (FMN) or flavinadenindinucleotide (FAD) in their active centres. The distinctive feature of flavoenzymes is their ability to transform single-electron transfer into a two-electron one. They play important roles in biological oxidation-reduction, hydroxylation, transhydrogenation, antioxidant protection, redox signalling, and other processes. Flavoenzymes also participate in biodegradation of toxic environmental pollutants and manifestation or neutralization of therapeutic activity/cytotoxicity of drugs or xenobiotics. Frequently, flavoenzymes are considered as drug targets. Taken together, these factors foster the permanent interest in the studies of flavoenzyme catalysis and its application in biomedicine, industries, and environmental protection. During last two decades, our studies were concentrated on the following issues: 1) the mechanisms of electron/hydride transfer in catalysis of flavoenzymes electrontransferases and transhydrogenases; 2) single- and two-electron reduction of quinones, nitroaromatics and other redox active organic compounds by mammalian, microbial or parasite flavoenzymes and their impact on their cytotoxicity. These studies were accompanied by intensive synthesis of above compounds; and 3) studies of prooxidant xenobiotics as inhibitors and subversive substrates for antioxidant mammalian or parasite FAD/SS and FAD/SS/SeS-containing enzymes.
Our main activities in 2017–2020 were as follows: a) characterization of interaction mechanism of quinones, nitroaromatics and aromatic N-oxides with possible target enzymes in bacteria (S. aureus flavohemoglobin, collaboration with Dr L. Baciou and F. Lederer, Université Paris-Sud, France), mammalian cells (neuronal NO synthase, collaboration with Dr J.-L. Boucher, Université Paris Descartes, France), and parasites (Plasmodium falciparum ferredoxin:NADP+ oxidoreductase, collaboration with Dr A. Aliverti, Universitá degli Studi di Milano, Italy); b) characterization of the mechanisms of two-electron reduction of quinones and nitroaromatics compounds by E. coli nitroreductase A and other nitroreductases (collaboration with Dr D. F. Ackerley, Victoria University of Wellington, New Zealand); c) evaluation of nitroaromatic compounds as inhibitors for Plasmodium falciparum glutathione reductase and Trypanosoma congolense trypanothione reductase in the context of development of antiplasmodial and antitrypanosomal agents (collaboration with Dr E. Davioud-Charvet, Université de Strasbourg, France, and Dr J. S. Blanchard, Albert Einstein College of Medicine, NY, USA); d) continuation of synthesis, studies of enzymatic single- and two-electron reduction and mammalian cell culture cytotoxicity studies of new polynitrobenzenes, nitrofurans, nitrothiophenes, and aromatic N-oxides (EU Structural Funds, Global Grant Measure, Grant No. 09.3.3-LMT-K-712-01-0058, 2018–2021). In 2020, we are continuing the studies of a potent antiplasmodial agent, 1,4-naphthoquinone plasmodione, and its derivatives (in collaboration with Dr E. Davioud-Charvet, Lithuanian-French Programme “Gilibert” , No. S-LZ-19-4). In addition, we have carried out the studies for antibacterial activity of aromatic di-N-oxide compounds used as single agents and in combination with conventional antibiotics, and participated in the studies of antibacterial photodynamic therapy.
SELECTED PUBLICATIONS
1. Nemeikaitė-Čėnienė, A., Šarlauskas, J., Misevičienė, L., Marozienė, A., Jonušienė, V., Lesanavičius, M., Čėnas, N. Aerobic cytotoxicity of aromatic N-oxides: the role of NAD(P)H:quinone oxidoreductase (NQO1). Int. J. Molec. Sci. 2020, 21: 8754.
2. Lesanavičius, M., Aliverti, A., Šarlauskas, J., Čėnas, N. Reactions of Plasmodium falciparum ferredoxin:NADP+ oxidoreductase with redox cycling xenobiotics: a mechanistic study. Int. J. Molec. Sci. 2020, 21: 3234.
3. Nemeikaitė-Čėnienė, A., Šarlauskas, J., Jonušienė, V., Marozienė, A., Misevičienė, L., Yantsevich, A. V., Čėnas, N. Kinetics of flavoenzyme-catalyzed reduction of tirapazamine derivatives: implications for their prooxidant cytotoxicity. Int. J. Molec. Sci. 2019, 20: 4602.
4. Marozienė, A., Lesanavičius, M., Davioud-Charvet, E., Aliverti, A., Grellier, P., Šarlauskas, J., Čėnas, N. Antiplasmodial activity of nitroaromatic compounds: correlation with their reduction potential and inhibitory action on Plasmodium falciparum glutathione reductase. Molecules. 2019, 24: 4509.
5. Moussaoui, M., Misevičienė, L., Anusevičius, Ž., Marozienė, A., Lederer, F., Baciou, L., Čėnas, N. Quinones and nitroaromatic compounds as subversive substrates of Staphylococcus aureus flavohemoglobin. Free Radic. Biol. Med. 2018, 123: 107–115.
Mechanisms of Antiplasmodial and Antitrypanosomal in vitro Activity of Nitroaromatic Compounds
We found that the in vitro antiplasmodial activity of a series of nitrobenzenes and nitrofurans increases with their single-electron reduction potential (E17), log D, and their ability to inhibit Plasmodium falciparum, but not human erythrocyte glutathione reductase. Ferredoxin:NADP+ oxidoreductase is expected to be the most active enzymatic reductant of nitroaromatics in parasite (Marozienė et al. Molecules. 2019, 24: 4509). Similarly, the in vitro activity of a series of 5-vinylquinoline-substituted nitrofurans against Trypanosoma b. brucei exhibited parallelism with their inhibitory activity against trypanothione reductase (Benitez et al. Chemija. 2020, 31: 111–117).
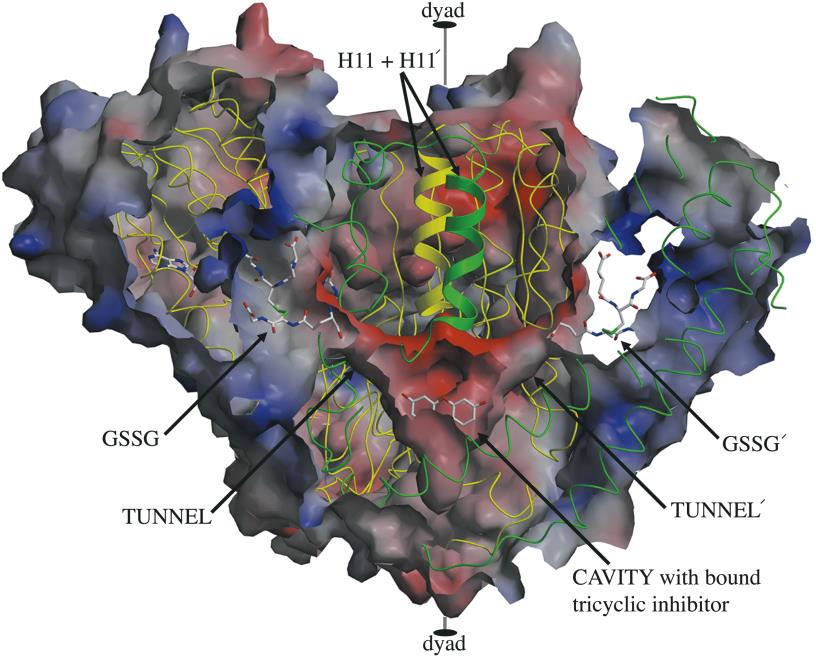
Structure of erythrocyte glutathione reductase with indicated domains of binding of substrates and inhibitors (Sarma et al. J. Mol. Biol. 2003, 328: 893–907)
Enzymatic Redox Reactions and Cytotoxicity of Aromatic N-oxides
3-Amino-1,2,4-benzotriazine-1,4-dioxide (tirapazamine, TPZ) is an anticancer agent selective for hypoxic cells. However, some of its derivatives may exert significant toxicity to normal (oxic) cells. We found that the aerobic cytotoxicity of a number of TPZ derivatives is well above that of quinones with similar E17 values, although their reactivity towards single-electron transferring flavoenzymes is similar. This was attributed to the potentiation of cytotoxicity of TPZ derivatives by cytochromes P-450 and NAD(P)H: quinone oxidoreductase (NQO1). The contribution of NQO1, which reduces TPZ derivatives in a mixed single- and two-electron way, is statistically significant (Nemeikaitė-Čėnienė et al. Int. J. Molec. Sci. 2019, 20: 4602; Int. J. Molec. Sci. 2020, 21: 8574).
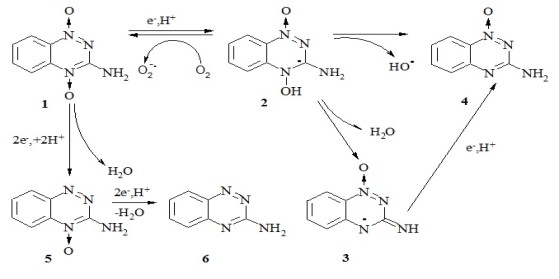
Scheme of single-electron reduction, redox cycling, and formation of metabolites of tirapazamine (1)
Antibacterial Activity of Aromatic di-N-oxides
Tirapazamine (TPZ) and its analogues were active with respect to both Gram-positive and -negative bacterial strains. The combination of TPZs with ciprofloxacin and nitrofurantoin (NFT) could generate additive and synergistic effects. These findings suggest that aromatic di-N-oxides may become valuable antibiotic complements (Polmickaitė-Smirnova et al. Appl. Sci. 2020, 10(12): 4062).
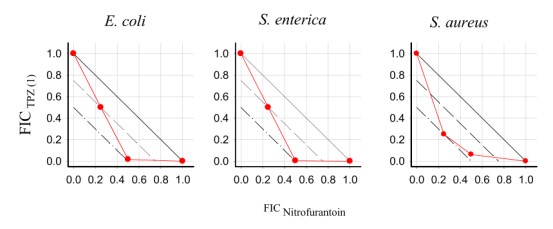
Isobolograms of combinatory action of TPZ and NFT (Polmickaitė-Smirnova et al., 2020).