 |
GINTARAS VALINČIUS
|
Protein Structure and Interactions in Phospholipid Membranes
The molecular organisation and function of biological membranes are essential to the understanding of living processes in general and the development of various biotechnological processes including molecular medicine in particular. Membrane proteins (MPs) represent almost 60% of pharmaceutical targets. However, despite their fundamental role, only 2% of the protein of known structure are that of MPs, and such lack of knowledge seriously affects understanding of the membrane protein functions slowing down the development of new diagnostic tools and therapies. The major difficulties and challenges for structural and functional studies of MPs arise from their instability outside a lipid bilayer environment, where specific hydrophobic and other molecular forces keep the protein in its native and active conformational state. Therefore, considerable efforts are directed towards the development of simplified but biologically relevant model membrane systems to study molecular processes in membranes.
Our group is specializing in the development of tethered bilayer membrane (tBLM) models. tBLMs are solid-supported phospholipid bilayers anchored to a surface via hydrophobic interactions between the molecular anchors and hydrophobic sheet of the membrane. The molecular anchors are synthetic thiolipids or silanes covalently attached to metal or metal-oxide surfaces. The anchors may contain hydrophilic fragments separating thiol/silane group and the glycerol backbone of the lipid, thus ensuring 1–2 nm thick water-reservoir between tethered bilayer and solid support. Alternatively, bilayers with no water sub-phase can be engineered. Recently, we developed an affordable and reproducible methodology for tBLM assembly using multilamellar vesicle fusion. We showed that such tBLMs are capable of reconstituting transmembrane proteins retaining their biological function. Membrane reconstituted proteins (peptides, oligomers) may be probed by the surface specific techniques, including surface plasmon resonance, vibrational spectroscopies and atomic force microscopy. Fine structural details revealing the molecular geometry of tBLMs are evaluated by the neutron reflectometry. Functional properties membranes with reconstituted protein complexes are accessible by the electrochemical impedance spectroscopy (EIS). The theoretical framework of EIS developed in our group allows a detailed analysis of protein membrane interactions as well as applications of tBLMs for bioanalysis.
SELECTED PUBLICATIONS
- Tumenas, S., Ragaliauskas, T., Penkauskas, T., Valanciute, A., Andriulevicius, F., Valincius, G. Solvent effects on composition and structure of thiolipid molecular anchors for tethering phospholipid bilayers. Applied Surface Science. 2020, 509: 145268.
- Talaikis, M., Valincius, G., Niaura, G. Potential-induced structural alterations in the tethered bilayer lipid membrane-anchoring monolayers revealed by electrochemical surface-enhanced Raman spectroscopy. J.Phys.Chem. C. 2020, 124(35): 19033–19045.
- Penkauskas, T., Zentelyte, A., Ganpule, S., Valincius, G., Preta, G. Pleiotropic effects of statins via interaction with the lipid bilayer: A combined approach. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2020, 1862(9): 183306.
- Raila, T., Ambrulevicius, F., Penkauskas, T., Jankunec, M., Meškauskas, T., Vanderah, D .J., Valincius, G. Clusters of protein pores in phospholipid bilayer membranes can be identified and characterized by electrochemical impedance spectroscopy. Electrochimica Acta. 2020, 364: 137179.
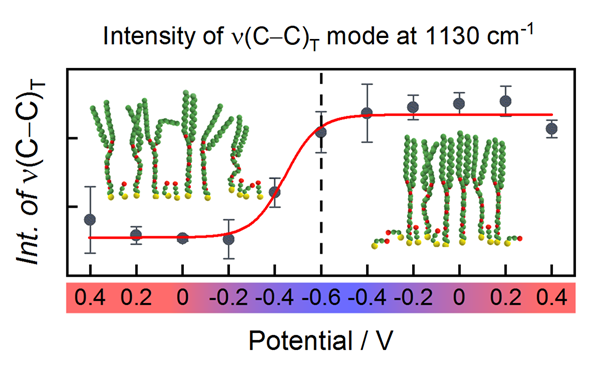
Properties and Function of Tethered Bilayer Membranes (tBLM), a biomimetic model of phospholipid membrane, depends on the structure of the self-assembled lipid-like monolayer that anchors tBLM to the surface. Electrode potential that can be adjusted via an external source is a physical variable, which can be used to fine-tune structure and possibly the function of tBLMs. In this project, we explored the potential-induced structural changes in monolayers composed of widely-used in tBLM design lipid-like long-chain WC14 molecules and short-chain hydrophilic backfiller 2 mercaptoethanol by the electrochemical surface-enhanced Raman spectroscopy. To infer the electric potential-induced changes in monolayer, the metal−adsorbate (i.e. Au–S and Au–O) spectral bands and C−C stretching vibrational mode of WC14's alkyl chains in all-trans configuration near 1130 cm-1 were analysed. Negative electric potential promotes the mobility of anchor molecules on the surface by decreasing the strength of metal−adsorbate bonding. At the same time, water pushes the hydrophobic WC14’s chains into phase-segregated clusters and in such a way minimizes the system’s energy. The clustering of molecular anchors has a detrimental effect on the integrity of tBLMs and their electrical insulation, therefore we propose the spectral band near 1130 cm-1 as a predictor of the functional properties of tBLMs. In general, our findings explain detrimental effects of negative going electric polarization of tBLMs empirically observed by a number of researchers before. This work was published in Talaikis et al. J. Phys. Chem. C. 2020, 124: 19033–19045).
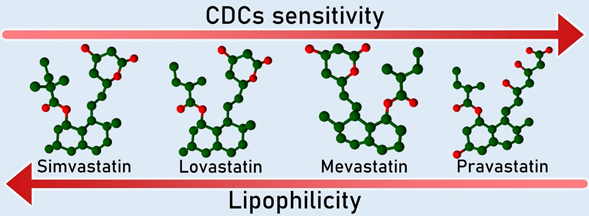
Pleiotropic Effects of Statins via Interaction with the Lipid Bilayers
Statins are selective inhibitors of cholesterol biosynthesis, used worldwide for cholesterol lowering in the primary and secondary prevention of cardiovascular diseases. Clinical trial studies indicated that the observed general benefits of statins appear to be greater than what might be expected from changes just in lipid levels, suggesting effects beyond cholesterol lowering (i.e. pleiotropic effects). Using a combined approach based on biophysical and biological methods, we demonstrate that lipophilic, but not hydrophilic statins are capable of reducing the damage caused by pneumolysin, a toxin of the cholesterol-dependent cytolysins (CDCs) family. This protection correlates with statins’ lipophilicity (expressed by the log P value) and capacity to interact with the lipid bilayer. Our data suggest that lipophilic statins associate with membranes and interfere with the ability of CDCs to bind to membrane cholesterol, influencing membrane lipid rafts organization. The ability to influence membrane lipid structure is one of the reported cholesterol-independent effects of statins. Evaluation of the capacity of statins to modulate membrane properties is an essential step for developing a correct therapeutic approach for cardiovascular diseases as well as for understanding the potential of this class of drugs in cancer therapy to increase tumour response to cytotoxic agents. The work was published in Penkauskas et al. BBA-Biomembranes. 2020, 1862(9): 183306.
Cluster formation is a widely-observed phenomenon, which results in unique sets of properties both in physical, chemical, and biological domains of nature. We found a mathematical description of clustering of membrane defects, specifically, of ion-conducting protein pores in tethered phospholipid bilayers. By invoking the Voronoi tessellation concept we demonstrate the possibility to distinguish between random and sparsely clustered patterns both in computer-generated and real-world systems using one single parameter σ, the standard deviation of the normalized Voronoi sector areas distribution. For random systems, σ0.54, and for clustered patterns σ>0.54. Because of a specific structure and dielectric properties of tethered bilayers, they can be characterized by an alternating current technique, electrochemical impedance spectroscopy (EIS). EIS measures macroscopic parameters of systems, and it is not a structural technique per se. However, we found the EIS spectra-derived quantitative metric ζ to be a diagnostic parameter that allows assessment of the distribution type (homogeneous, random or clustered) of defects at nanometre level. One of the most interesting findings of the current study is the fact that the EIS derived ζ parameter is sensitive to an average size of the defects, thus, enabling a purely electrochemical methodology to access fine structural information such as the size of incomplete protein pores in phospholipid bilayers. Overall, our results demonstrate a fundamental property of the macroscopic technique, electrochemical impedance spectroscopy, to probe structural arrangement of defects with sizes between 0.5 nm to 25.5 nm located in a thin, 2 nm thick phospholipid dielectric layer. This project is a part of collaboration efforts with Dr. Tadas Meškauskas’ group from the Faculty of Mathematics and Informatics at Vilnius University. It was published in Raila et al. Electrochimica Acta, 2020, 364: 137–179.
