|
GINTAUTAS TAMULAITIS |
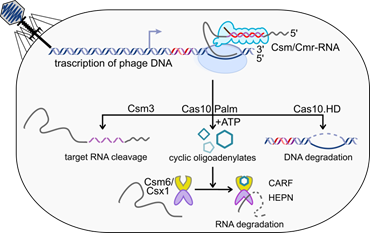
Fig.1. Type III CRISPR-Cas immunity mechanism. |
Signalling in Prokaryotic Antiviral Defence
Cyclic mono- and di-ribonucleotides are widely employed for controlling various biological processes by eukaryotes and especially prokaryotes. Purine ribonucleotides serve not only as building blocks for RNA, universal currency of energy and components of coenzymes such as NAD(P)+, FAD or CoA, but are also assembled into signalling molecules. Both prokaryotes and eukaryotes employ cyclic AMP (cAMP) and cGMP as key second messengers in a variety of biological processes, including quorum sensing, energy homeostasis, neuronal signalling, and muscle relaxation. Prokaryotes also use a variety of cyclic dinucleotides – c-di-AMP, c-di-GMP, and 3’3’-cGAMP – as second messengers for biofilm formation, virulence and regulation of bacterial cell cycle. Recent studies in bacteria have reported that various cyclic oligonucleotides are used as signalling molecules in bacterial antiviral defence systems of Type III CRISPR-Cas and CBASS (cyclic-oligonucleotide-based anti-phage signalling systems). As these systems are widespread and abundant, this suggests that signalling is widely used in prokaryotic antiviral defence.
Our group has pioneered by elucidating the interference mechanism of the Type III CRISPR-Cas immunity 1–4. We revealed that to provide immunity against invading nucleic acids in prokaryotes, Type III CRISPR-Cas system combines transcription-dependent DNA degradation by crRNA guided Csm or Cmr complex [1–3] with the cyclic oligoadenylates (cAn)-dependent immunity pathway [3–5]. In response to the viral RNA binding the Csm/Cmr complex synthesizes cAn molecules of various ring size (n=2–6) [4,5]. The cA4 or cA6 acts as a signalling molecule that binds to the CARF domain sensor of the stand-alone Csm6 or Csx1 proteins and allosterically activates the non-specific ribonucleolytic activity of their HEPN effector domains [4,5]. Bioinformatics analysis revealed that the sensor CARF and SAVED (a divergent version of CARF) domains are found fused with different enzymatic or non-enzymatic effector domains of Type III CRISPR-Cas associated or CRISPR-Cas unrelated proteins. We aim to understand the molecular and structural mechanisms by which CARF/SAVED domain-containing proteins are involved in bacterial immunity or possibly other cell processes.
SELECTED PUBLICATIONS
- Kazlauskiene, M., Tamulaitis, G., Kostiuk, G., Venclovas, Č., Siksnys, V. Spatiotemporal control of type III-A CRISPR-Cas immunity: coupling DNA degradation with the target RNA recognition. Molecular Cell. 2016, 62: 295–306.
- Tamulaitis, G., Venclovas, Č, Siksnys V. Type III CRISPR-Cas immunity: major differences brushed aside. Trends in Microbiology. 2017, 25(1): 49–61.
- Mogila, I., Kazlauskiene, M., Valinskyte, S., Tamulaitiene, G., Tamulaitis, G., Siksnys, V. Genetic dissection of the type III-A CRISPR-Cas system Csm complex revealsroles of individual subunits. Cell Reports. 2019, 26(10): 2753–2765, e4.
- Kazlauskiene, M., Kostiuk, G., Venclovas, Č., Tamulaitis, G., Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems. Science. 2017, 357: 605–609.
- Smalakyte, D., Kazlauskiene, M., Havelund, J.F., Rukšėnaitė, A., Rimaite, A., Tamulaitiene, G., Færgeman, N. J., Tamulaitis, G., Siksnys, V. Type III-A CRISPR-associated protein Csm6 degrades cyclic hexa-adenylate activator using both CARF and HEPN domains. Nucleic Acids Research. 2020, 48(16): 9204–9217.